Impressions of the phycological field trip
to the island of Hiddensee in the Baltic Sea,
spring 2010
In spring 2010 I've participated on the phycological excursion to the island of Hiddensee in the Baltic Sea with the Menzel lab of the IZMB.
We were accomodated at the Biological Station Hiddensee
of the Ernst Moritz Arndt University of Greifswald, which provided not only facilities for accomodation, but also a course room with basic
equipment for specimen preparation and observation and multimedia access.
In preparation for the course each of the participants had chosen a topic or a algae clade to focus on during the excursion.
For my part I covered the realm of chlorophytes and especially the clade of the Characeae which are known to thrive in the brackish waters of the Baltic Sea.
Below you will find an introduction to the clade of chlorophytes in PDF file format for download which was originally presented during the course.
During the course specimen were collected from their habitats (shallow waters of the shoreline and the 'Bodden') and then kept at the seawater aquarium of the station. Unfortunately, the species diversity of available chlorophytes at the shores of the island was quite limited, so I focused mainly on only three genera: Ulva/Enteromorpha, Cladophora and Chara. On selected species several staining methods, with or without prior fixation, were applied.
For the stainings the object was placed with a drop of sea water on a glass slide and covered with a glass cover-slide. Then a drop of the staining solution was placed beside the cover slide and this drop was sucked through the medium between the object and cover slide by pressing a piece of paper (filter or blotting paper) to the opposite side of the covered object. These first steps showed that the right volume of staining solution must be found empirically.
Dyes used in biological staining techniques can be distinguished and classified by several criteria, based on the differing properties of the chemicals:
Beside the chemical composition and classification of dyes, like the distinction of azo, phenazine, phenothiazine or aniline dyes, and the resulting color of these agents, the most important property for biological applications is the ionic condition of the dye. So, all the staining agents can be divided in basic, acidic and neutral dyes and the grade of ionisation determines on which biological components the dye acts on. Moreover, most of the used dyes, especially the aniline dyes can be divided in metachromatic and orthochromatic ones, a definition of these properties is, for example, given by Padday (1969):
Definition of metachromasy (metachromasia): ".... a characteristic reversible colour change that any dye may undergo by virtue of a change in its environment not involving chemical reaction of the dye", cited in Scott (1970).
Thus, staining dyes which change their color due to interaction with the stained material of the object are called metachromatic and those which keep their color unchanged are referred to as orthochromatic.
As I had beforehand no special experience in staining green algae, one conclusion that must be drawn from the multiple efforts to yield representative results is that:
"Algae staining isn't easy!"
There are several reasons, that green algae behave in parts very differently from what is known from classical staining of other botanical material, like sections of stems or leaves of higher plants. First of all, the algae cells are much more fragile than, for example, woody plants. The cells are often solitary or bound to single-celled layers and are quite sensitive to changes in their environment, especially to changes in pH, ionic conditions, carbondioxide or oxygen concentration. Thus, under culturing conditions the choosen medium is of utmost importance. In our aquarium of the biological station the natural seawater of the Baltic Sea was used, which is of lower salt content than water of other seas or oceans like the North Sea or the Atlantic. The water of the eastern coast of the island, the so-called Bodden, is even more brackish and imposes special conditions on the submersed algae and plants growing there. The medium is not only important for the proper handling of the algae species, it does also interact, often in a unpredictable and unforeseen way, with the staining dyes, which are often recommended for use in defined solutions. As an example may serve the made experience, that congo red was not stable in the salt water or resulted in somewhat diffuse staining behaviour and could not be used to yield stained living cells (see Fig. 12). Beside the fragility of the algae cells, another difficulty, compared to the staining methods of higher plants, arise from the fact that algae incorporate unusual polysaccharides, like mannans or uronic acids, into their cell walls. These polymers are often characteristic for a taxon and cellulose can be wholly absent, e.g. see text-books of Lee (2008) or van den Hoek et. al. (1995). Thus, the retention of dyes within the special polymeric structures or the passage through the molecular layers of the algae cell walls can differ substantially from that of cellulosic cell walls. Therefore, staining methods designed for differentiation of plant cell walls might be inappropiate in algae staining. On the other hand, certain basophilic or acidophilic dyes might be suitable to demonstrate just this occurrence of unusal acidic or basic functional groups within the polysaccharides of the cell wall. In my attempts of proper cell wall staining, best results were obtained by application of toluidine blue-alcohol and safranine, although the latter dye often resulted in whole cell staining, where the cell walls were stained together with the nuclei and the cytoplasm, but appeared quite differentiated and distinct. The safranine staining of Cladophora exhibited also some, quite peculiar effects (see Figs. 09, 10 and 34-36), worthwhile of further investigation, possibly by an amended methodology. With respect to the color intensity, the safranine appeared to accumulate at the cell poles, especially at ramifications near the apical parts of the cells. In living cells this dye accumulation pointed at a possible reinforcement of the cell wall at these positions (Figs. 09 and 10), while in fixed, dead cells the color intensity was strongest within the polar cytoplasmic portion, seen as a dye concentration at the basal region in the cells of the regular filament and an apical and a basal accumulation at ramifications (Fig. 34-36). This highly demarcated dye concentration might be artifactual, but could also hint to special compounds, most probable of acidic (i.e. basophilic) nature, which might be prominently present in this region of the cell. Such compounds, either proteins or polysaccharides, might exhibit structural significance when either considering that these polar parts might be involved in some sort of cell to cell communication or take part in ongoing cell wall synthesis. As it is known from the green alga Acetabularia, that RNA associated proteins, the so-called ribonucleoproteins (RNP), do play a role in morphogenetic changes by accumulating in the tip of the cell, it could be thought of a similar role for such RNP's in the branching process of Cladophora, so that such acidic molecules possibly concentrate in the branching regions of the cell, either to provide matrices for protein synthesis or even to participate in catalytic turnover.
An additional restriction imposed on staining dyes does not only apply to green algae but also to the green parts of all higher plants: It is the occurrence of chloroplasts and the pigments contained within these organelles. Many dyes stain, metachromatic or orthochromatic (see above) with a green color, which turns out to be of little use in the presence of bright green chlorophyll. Thus, applied dyes should be scrutinized especially for their metachromatic effects. For example, acridine orange is commonly used for staining nucleic acids, whereby metachromatic effects lead to a fluorescent red staining of RNA and a fluorescent green color of DNA. Such functionality appears to be of little value when green algae are stained, where nearly every cell contains prominent chloroplasts. In an apparent contradiction to this consideration, I made the experience that acridine orange could nevertheless be used as an orthochromatic dye in staining parts of Enteromorpha sp., as the dye exhibited a good retardation within the cell's walls and cytoplasm and thus was useful as a general contrasting agent (see Figs. 55 and 56) . Similarly, the orange colored dye chrysoidine was useful in contrasting the usually transparent and colorless cytoplasm from chloroplasts and exhibited similar effects as safranine (see Fig. 08). While the chloroplasts themselves are usually good visible under bright-field microscopy (see Fig. 16), the white, starch storing bodies contained within them are sometimes less prominent. These so-called pyrenoids can be better differentiated by staining with a iodine solution, mostly employed as the so-called Lugol's solution, a mixture of I2 (elementary iodine) and KI (potassium iodide) solved in water. Under benign circumstances the Lugol stain might be even able to distiguish between amylopectin and amylose, the two main components of starch. In such cases amylopectins are stained reddish-violet, while amylose tends to exhibit a more blueish tinge. The iodine staining of the green algae collected during the coursework exhibited good results and showed, that the pyrenoids of Enteromorpha sp. seem to contain a higher content of amylopectin (see Figs. 32 and 33), while the pyrenoids of Cladophora sp. appeared slightly more blueish, hinting at a higher amylose content (see Figs. 49 and 50). For the staining of nuclei several dyes are known to yield good results, most of them either interacting with the acidic (phosphates) or the basic groups (nucleotide bases) of the nuclear acids. In my staining procedures I've obtained the best results with a hematoxylin-alcohol stain, which led to a reddish-brown coloring of the nuclei (see Figs. 11, 54 and 64). As seen in the figures, the nuclei exhibited differing color intensity and therefore it must be assumed that this procedure is highly sensitive to the length of exposure to the stain. Although in some samples even the nucleoli were clearly visible, the hematoxylin staining can certainly be improved, if some empirical data is collected on the time-dependent intensity of the stain.
Fixation is also a very important factor for obtaining good staining results. We used primarily alcoholic (methanol and ethanol) fixation, but formaldehyde was also deployed. Success of certain fixation procedures appeared to me quite empirical and choice of the right fixation method needed to be tested in each case. Sometimes, the alcoholic treatment appeared to facilitate the uptake of the dye molecules, while in other instances it was more important that the cytoplasmic material was fixated. In particular, the application of some sort of fixation seemed to be the superior approach when cytoplasmic structures were to be stained.
Obviously, I had used only a limited number of the coloring agents from the plentiful arsenal of dyes which was to our hands. Many dyes turned out not to be of practical value or were simply not examined properly due to the lack of time, in particular as each dye was tested on living and on fixed, dead cells.
So, the experienced phycologist might excuse the often sub-optimal staining results presented in the images below. Unfortunately, there are little standard procedures described for staining green algae and a standardized method, like for example the Giemsa method used for staining blood cells, is lacking altogether. Probably, the development of such a quantifiable, standardized staining method would be a recommended, but challenging, task for upcoming resarch work, especially in foresight of the more important role algae will play in human industrial development (see for example my compilation about algae derived biofuels).
Apart from the images of the course work, I've tried to capture some impressions of the island itself and especially its diverse botanic habitats. These images were taken by my mobile's phone cam, so please, do not expect the most brilliant photographs...
Although Hiddensee is a quite small island of about 17 km length and 3,7 km width at its broadest point (I've surrounded most of the island on foot in just about 6 h), it is composed of several different habitats, like heath, bogs, salt and brackish waters, dense forest, meadow lands, salty marshes and bushy areas. Moreover, the North-Eastern part of the island is subjected to extreme changes in land pattern by the ever-perturbing tidal forces, which destroys land in one place (mostly the Northern tip) and recreates it in other locations anew, a process which has produced two sandspits in the North-East. These spits are composed from sediment and the elder one ("Alter Bessin") is about 400 years old, while the younger one ("Neuer Bessin") is just about 100 years old and grows by 30-60 metres a year.
All these environments give various plant and animal species ground to thrive on and a small excursion led by Dr. Blindow of the Biological Station to the heath lands south of Vitte proved to be very insightful. Certainly, any interested botanist will find plenty of opportunities to make interesting discoveries on this small island.
References:
General textbooks:
WWW resources:
In preparation for the course each of the participants had chosen a topic or a algae clade to focus on during the excursion.
For my part I covered the realm of chlorophytes and especially the clade of the Characeae which are known to thrive in the brackish waters of the Baltic Sea.
Below you will find an introduction to the clade of chlorophytes in PDF file format for download which was originally presented during the course.
During the course specimen were collected from their habitats (shallow waters of the shoreline and the 'Bodden') and then kept at the seawater aquarium of the station. Unfortunately, the species diversity of available chlorophytes at the shores of the island was quite limited, so I focused mainly on only three genera: Ulva/Enteromorpha, Cladophora and Chara. On selected species several staining methods, with or without prior fixation, were applied.
For the stainings the object was placed with a drop of sea water on a glass slide and covered with a glass cover-slide. Then a drop of the staining solution was placed beside the cover slide and this drop was sucked through the medium between the object and cover slide by pressing a piece of paper (filter or blotting paper) to the opposite side of the covered object. These first steps showed that the right volume of staining solution must be found empirically.
Dyes used in biological staining techniques can be distinguished and classified by several criteria, based on the differing properties of the chemicals:
Beside the chemical composition and classification of dyes, like the distinction of azo, phenazine, phenothiazine or aniline dyes, and the resulting color of these agents, the most important property for biological applications is the ionic condition of the dye. So, all the staining agents can be divided in basic, acidic and neutral dyes and the grade of ionisation determines on which biological components the dye acts on. Moreover, most of the used dyes, especially the aniline dyes can be divided in metachromatic and orthochromatic ones, a definition of these properties is, for example, given by Padday (1969):
Definition of metachromasy (metachromasia): ".... a characteristic reversible colour change that any dye may undergo by virtue of a change in its environment not involving chemical reaction of the dye", cited in Scott (1970).
Thus, staining dyes which change their color due to interaction with the stained material of the object are called metachromatic and those which keep their color unchanged are referred to as orthochromatic.
As I had beforehand no special experience in staining green algae, one conclusion that must be drawn from the multiple efforts to yield representative results is that:
"Algae staining isn't easy!"
There are several reasons, that green algae behave in parts very differently from what is known from classical staining of other botanical material, like sections of stems or leaves of higher plants. First of all, the algae cells are much more fragile than, for example, woody plants. The cells are often solitary or bound to single-celled layers and are quite sensitive to changes in their environment, especially to changes in pH, ionic conditions, carbondioxide or oxygen concentration. Thus, under culturing conditions the choosen medium is of utmost importance. In our aquarium of the biological station the natural seawater of the Baltic Sea was used, which is of lower salt content than water of other seas or oceans like the North Sea or the Atlantic. The water of the eastern coast of the island, the so-called Bodden, is even more brackish and imposes special conditions on the submersed algae and plants growing there. The medium is not only important for the proper handling of the algae species, it does also interact, often in a unpredictable and unforeseen way, with the staining dyes, which are often recommended for use in defined solutions. As an example may serve the made experience, that congo red was not stable in the salt water or resulted in somewhat diffuse staining behaviour and could not be used to yield stained living cells (see Fig. 12). Beside the fragility of the algae cells, another difficulty, compared to the staining methods of higher plants, arise from the fact that algae incorporate unusual polysaccharides, like mannans or uronic acids, into their cell walls. These polymers are often characteristic for a taxon and cellulose can be wholly absent, e.g. see text-books of Lee (2008) or van den Hoek et. al. (1995). Thus, the retention of dyes within the special polymeric structures or the passage through the molecular layers of the algae cell walls can differ substantially from that of cellulosic cell walls. Therefore, staining methods designed for differentiation of plant cell walls might be inappropiate in algae staining. On the other hand, certain basophilic or acidophilic dyes might be suitable to demonstrate just this occurrence of unusal acidic or basic functional groups within the polysaccharides of the cell wall. In my attempts of proper cell wall staining, best results were obtained by application of toluidine blue-alcohol and safranine, although the latter dye often resulted in whole cell staining, where the cell walls were stained together with the nuclei and the cytoplasm, but appeared quite differentiated and distinct. The safranine staining of Cladophora exhibited also some, quite peculiar effects (see Figs. 09, 10 and 34-36), worthwhile of further investigation, possibly by an amended methodology. With respect to the color intensity, the safranine appeared to accumulate at the cell poles, especially at ramifications near the apical parts of the cells. In living cells this dye accumulation pointed at a possible reinforcement of the cell wall at these positions (Figs. 09 and 10), while in fixed, dead cells the color intensity was strongest within the polar cytoplasmic portion, seen as a dye concentration at the basal region in the cells of the regular filament and an apical and a basal accumulation at ramifications (Fig. 34-36). This highly demarcated dye concentration might be artifactual, but could also hint to special compounds, most probable of acidic (i.e. basophilic) nature, which might be prominently present in this region of the cell. Such compounds, either proteins or polysaccharides, might exhibit structural significance when either considering that these polar parts might be involved in some sort of cell to cell communication or take part in ongoing cell wall synthesis. As it is known from the green alga Acetabularia, that RNA associated proteins, the so-called ribonucleoproteins (RNP), do play a role in morphogenetic changes by accumulating in the tip of the cell, it could be thought of a similar role for such RNP's in the branching process of Cladophora, so that such acidic molecules possibly concentrate in the branching regions of the cell, either to provide matrices for protein synthesis or even to participate in catalytic turnover.
An additional restriction imposed on staining dyes does not only apply to green algae but also to the green parts of all higher plants: It is the occurrence of chloroplasts and the pigments contained within these organelles. Many dyes stain, metachromatic or orthochromatic (see above) with a green color, which turns out to be of little use in the presence of bright green chlorophyll. Thus, applied dyes should be scrutinized especially for their metachromatic effects. For example, acridine orange is commonly used for staining nucleic acids, whereby metachromatic effects lead to a fluorescent red staining of RNA and a fluorescent green color of DNA. Such functionality appears to be of little value when green algae are stained, where nearly every cell contains prominent chloroplasts. In an apparent contradiction to this consideration, I made the experience that acridine orange could nevertheless be used as an orthochromatic dye in staining parts of Enteromorpha sp., as the dye exhibited a good retardation within the cell's walls and cytoplasm and thus was useful as a general contrasting agent (see Figs. 55 and 56) . Similarly, the orange colored dye chrysoidine was useful in contrasting the usually transparent and colorless cytoplasm from chloroplasts and exhibited similar effects as safranine (see Fig. 08). While the chloroplasts themselves are usually good visible under bright-field microscopy (see Fig. 16), the white, starch storing bodies contained within them are sometimes less prominent. These so-called pyrenoids can be better differentiated by staining with a iodine solution, mostly employed as the so-called Lugol's solution, a mixture of I2 (elementary iodine) and KI (potassium iodide) solved in water. Under benign circumstances the Lugol stain might be even able to distiguish between amylopectin and amylose, the two main components of starch. In such cases amylopectins are stained reddish-violet, while amylose tends to exhibit a more blueish tinge. The iodine staining of the green algae collected during the coursework exhibited good results and showed, that the pyrenoids of Enteromorpha sp. seem to contain a higher content of amylopectin (see Figs. 32 and 33), while the pyrenoids of Cladophora sp. appeared slightly more blueish, hinting at a higher amylose content (see Figs. 49 and 50). For the staining of nuclei several dyes are known to yield good results, most of them either interacting with the acidic (phosphates) or the basic groups (nucleotide bases) of the nuclear acids. In my staining procedures I've obtained the best results with a hematoxylin-alcohol stain, which led to a reddish-brown coloring of the nuclei (see Figs. 11, 54 and 64). As seen in the figures, the nuclei exhibited differing color intensity and therefore it must be assumed that this procedure is highly sensitive to the length of exposure to the stain. Although in some samples even the nucleoli were clearly visible, the hematoxylin staining can certainly be improved, if some empirical data is collected on the time-dependent intensity of the stain.
Fixation is also a very important factor for obtaining good staining results. We used primarily alcoholic (methanol and ethanol) fixation, but formaldehyde was also deployed. Success of certain fixation procedures appeared to me quite empirical and choice of the right fixation method needed to be tested in each case. Sometimes, the alcoholic treatment appeared to facilitate the uptake of the dye molecules, while in other instances it was more important that the cytoplasmic material was fixated. In particular, the application of some sort of fixation seemed to be the superior approach when cytoplasmic structures were to be stained.
Obviously, I had used only a limited number of the coloring agents from the plentiful arsenal of dyes which was to our hands. Many dyes turned out not to be of practical value or were simply not examined properly due to the lack of time, in particular as each dye was tested on living and on fixed, dead cells.
So, the experienced phycologist might excuse the often sub-optimal staining results presented in the images below. Unfortunately, there are little standard procedures described for staining green algae and a standardized method, like for example the Giemsa method used for staining blood cells, is lacking altogether. Probably, the development of such a quantifiable, standardized staining method would be a recommended, but challenging, task for upcoming resarch work, especially in foresight of the more important role algae will play in human industrial development (see for example my compilation about algae derived biofuels).
Apart from the images of the course work, I've tried to capture some impressions of the island itself and especially its diverse botanic habitats. These images were taken by my mobile's phone cam, so please, do not expect the most brilliant photographs...
Although Hiddensee is a quite small island of about 17 km length and 3,7 km width at its broadest point (I've surrounded most of the island on foot in just about 6 h), it is composed of several different habitats, like heath, bogs, salt and brackish waters, dense forest, meadow lands, salty marshes and bushy areas. Moreover, the North-Eastern part of the island is subjected to extreme changes in land pattern by the ever-perturbing tidal forces, which destroys land in one place (mostly the Northern tip) and recreates it in other locations anew, a process which has produced two sandspits in the North-East. These spits are composed from sediment and the elder one ("Alter Bessin") is about 400 years old, while the younger one ("Neuer Bessin") is just about 100 years old and grows by 30-60 metres a year.
All these environments give various plant and animal species ground to thrive on and a small excursion led by Dr. Blindow of the Biological Station to the heath lands south of Vitte proved to be very insightful. Certainly, any interested botanist will find plenty of opportunities to make interesting discoveries on this small island.
References:
Scott, J. E. (1970) Histochemistry of Alcian Blue 1. Metachromasia of Alcian Blue, Astrablau and other Cationic Phthalocyanin Dyes, Histochemie, 21, 277-285
General textbooks:
van den Hoek, C., Mann, D. G., Jahns, H. M. 'Algae: an introduction to phycology', Cambridge University Press 1995
Lee, R. E. 'Phycology', 4th Edition, Cambridge University Press 2008
Kornmann, P. & Sahling, P.-H. (1977) 'Meeresalgen yon Helgoland, Benthische Grün-, Braun- und Rotalgen', Helgoländer wiss. Meeresunters. 29, 1-289
Lee, R. E. 'Phycology', 4th Edition, Cambridge University Press 2008
Kornmann, P. & Sahling, P.-H. (1977) 'Meeresalgen yon Helgoland, Benthische Grün-, Braun- und Rotalgen', Helgoländer wiss. Meeresunters. 29, 1-289
WWW resources:
Algaebase, a seaweed database founded by Ireland's Higher Education Authority,
the European Union and the Ryan Institute, as well as various other contributors.
Hiddensee, Wikipedia.org
Hiddensee GmbH, Island Tourist Information (German)
Hiddensee, Wikipedia.org
Hiddensee GmbH, Island Tourist Information (German)
Excursion Diary
You can use the following calendar for navigation to the single course days:
Sat. 29.05.10, Day 2
- short trip to the Nordkap
- encountered specimen: Cladophora, Enteromorpha, Ectocarpus, unidentified brown alga (Chordaria ?)
- location: shallow, stony part of the litoral, influenced by smooth waves
- short trip to the Nordkap
- encountered specimen: Cladophora, Enteromorpha, Ectocarpus, unidentified brown alga (Chordaria ?)
- location: shallow, stony part of the litoral, influenced by smooth waves
Sun. 30.05.10, Day 3
- start of laboratory activities:
- photographs and sketches of Cladophora and Enteromorpha
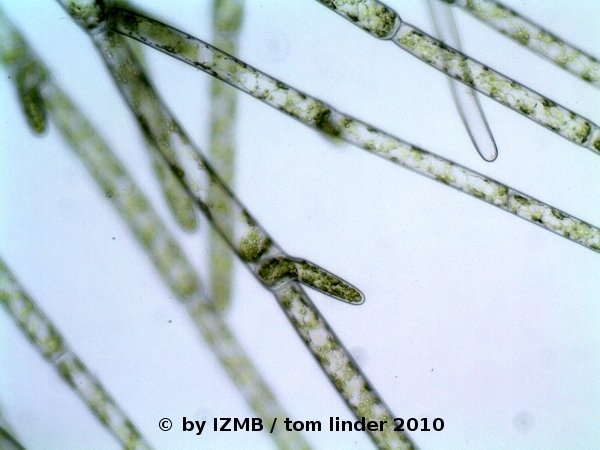
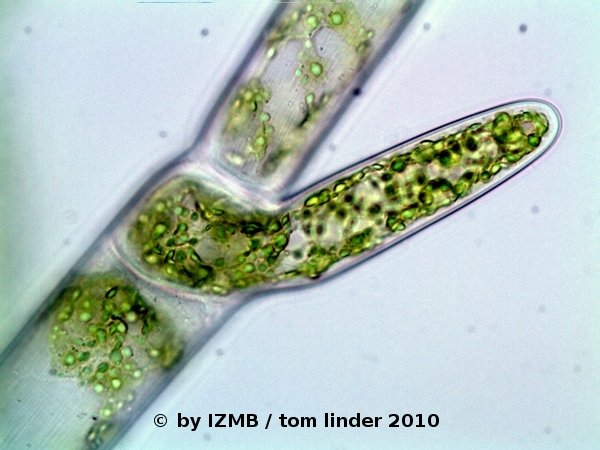
Fig. 1: Cladophora sp., left: magnification 100x, right: magnification 400x


Fig. 2: Cladophora sp., left: magnification 160x, right: magnification 400x, DIC, © Sebastian Hess 2010
- start of laboratory activities:
- photographs and sketches of Cladophora and Enteromorpha


Fig. 1: Cladophora sp., left: magnification 100x, right: magnification 400x


Fig. 2: Cladophora sp., left: magnification 160x, right: magnification 400x, DIC, © Sebastian Hess 2010
Mon. 31.05.10, Day 4
Laboratory course:
- Imaging and drawings of Cladophora at the binocular, in particular in order to illustrate the main filamentous trunk (stalk) and the pseudo-dichotomous ramifications
- Stainings with toluidine-borax, picric acid/aniline blue, afterwards digital imaging of the objects and staining with hematoxylin/alcohole, acidic hematoxyline and congo red overnight.
- Toluidine-borax is often used in electron microscopy for pre-staining thick/semi-thin sections, in order to give an overview of the preparation. The borax is used to keep the solution alkaline, facilitating thereby the penetration of epoxy mounted specimen (e.g. see Toluidine blue staining protocol, IHC World LLC, Woodstock, MD, USA).
- Fig. 3 highlights the yellowish-green appearance of the alga and its organization into multiple, central stalk or 'trunk'-like filaments, from which twig-like branches ramify
- Especially the right image in Fig. 4 demonstrates the acropetal growth mode of Cladophora filaments, as the length of the primary branching cells on a single filament reveals also their age, i.e. the lower, longer one is the oldest, while the upper, smaller one has grown out least.
- Figs. 5 and 6 show the results of the toluidine-borax staining.
This staining method clearly demonstrates a general difficulty encountered when staining the fragile algae cells:
It seems a matter of empirical 'try and error' to find the right amount of dye (the right volume of dye solution) to yield optimal staining 'strength'.
So the bottom of the alga fragment on the left in Fig. 5 appears already 'over-stained', while the upper part is still transparent and unstained.
By zooming into the well stained sections of the preparation (Fig. 6), an orange-red coloring of the cell interior, due to a metachromatic effect, is revealed. According to the theory of toluidine stains, these orange stained parts mark the cytoplasm, possibly rich in RNA.
Blue stained strands or patches are also visible, especially seen on the image to the right of Fig. 6.
Again according to the theory, these blue regions should mark DNA-rich areas (nuclei) of the cell.
- Obviously, the cell's walls are stained purplish-violet and seem to contain a high content of dye.
This retardation of the dark dye in the cell wall appeared quite disturbing to me, because it obstructed the overall observation of the cell's interior.
Moreover, the tendency to 'over-stain' gave the whole preparation a diffuse, smudged appearance.
So in following staining procedures I refrained from further attempts of toluidine-borax stains.
This should not state that the stain is of no use at all, but certainly more time, a systematic effort and the application of accompanying methods for verification of the molecular foundation for the staining effects should be employed, to give a more differentiated stain.
Possibly, much smaller alga fragments, stained with minute amounts of dye will yield better results.
- Apart from the staining, the image on the left of Fig. 6 highlights another anatomic feature of Cladophora, called pseudo-dichotomous ramification.
Such ramifications occur when branching cells grow out of an intercalary cell.
In opposite to true dichotomy, the filament does not ramify into two new branches, instead each branch is formed by a distinct cell, growing out into a new filament.
Here, at least five branches are depicted.


Fig. 3: Cladophora sp., left: magnification 100x, right: magnification 320x.


Fig. 4: Cladophora sp., left: magnification 100x, right: magnification 400x.
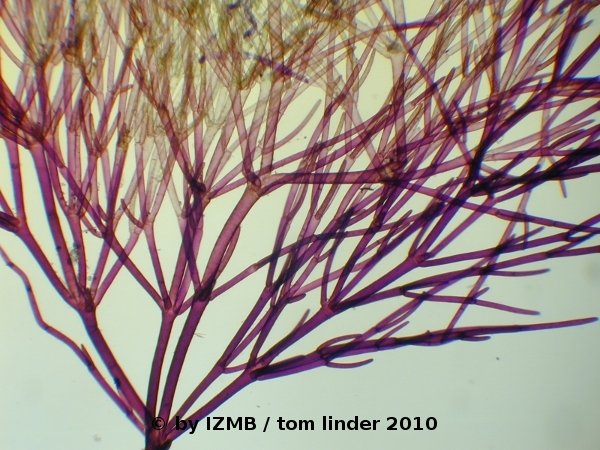
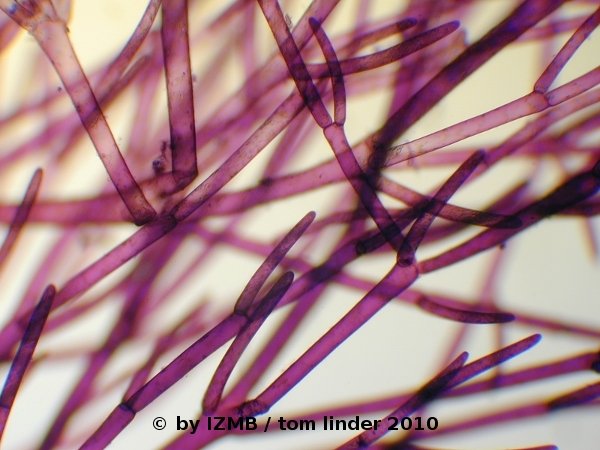
Fig. 5: Cladophora sp., left: magnification 40x, right: magnification 100x, toluidine-borax staining
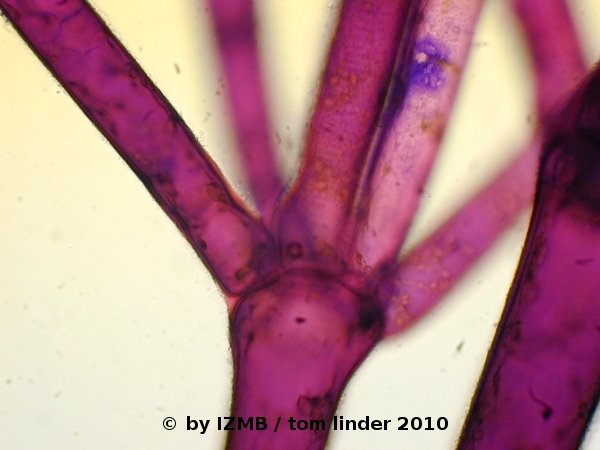
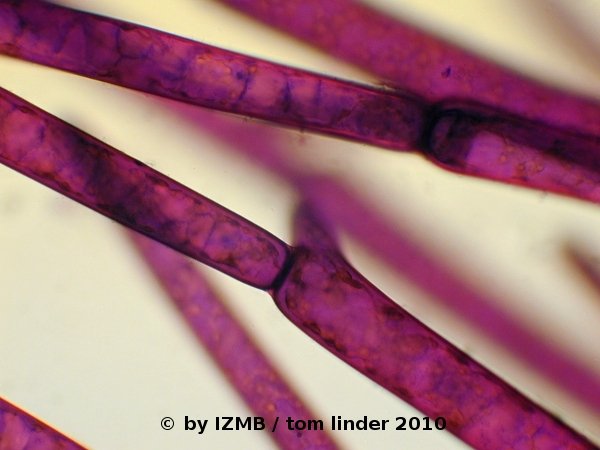
Fig. 6: Cladophora sp., left: magnification 40x, right: magnification 100x, toluidine-borax staining

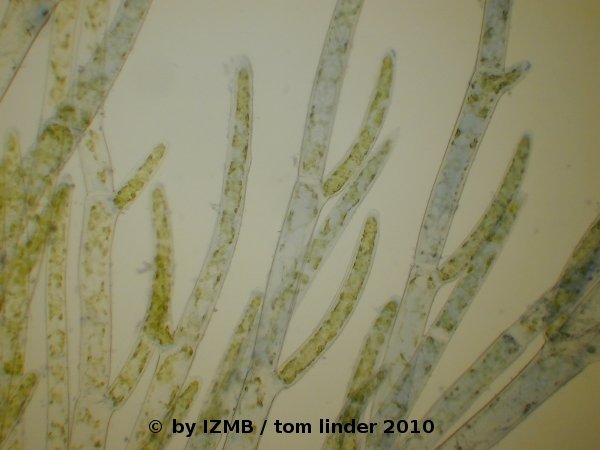
Fig. 7: Cladophora sp., left: magnification 40x, right: magnification 100x, picric acid/aniline blue staining
Laboratory course:
- Imaging and drawings of Cladophora at the binocular, in particular in order to illustrate the main filamentous trunk (stalk) and the pseudo-dichotomous ramifications
- Stainings with toluidine-borax, picric acid/aniline blue, afterwards digital imaging of the objects and staining with hematoxylin/alcohole, acidic hematoxyline and congo red overnight.
- Toluidine-borax is often used in electron microscopy for pre-staining thick/semi-thin sections, in order to give an overview of the preparation. The borax is used to keep the solution alkaline, facilitating thereby the penetration of epoxy mounted specimen (e.g. see Toluidine blue staining protocol, IHC World LLC, Woodstock, MD, USA).
- Fig. 3 highlights the yellowish-green appearance of the alga and its organization into multiple, central stalk or 'trunk'-like filaments, from which twig-like branches ramify
- Especially the right image in Fig. 4 demonstrates the acropetal growth mode of Cladophora filaments, as the length of the primary branching cells on a single filament reveals also their age, i.e. the lower, longer one is the oldest, while the upper, smaller one has grown out least.
- Figs. 5 and 6 show the results of the toluidine-borax staining.
This staining method clearly demonstrates a general difficulty encountered when staining the fragile algae cells:
It seems a matter of empirical 'try and error' to find the right amount of dye (the right volume of dye solution) to yield optimal staining 'strength'.
So the bottom of the alga fragment on the left in Fig. 5 appears already 'over-stained', while the upper part is still transparent and unstained.
By zooming into the well stained sections of the preparation (Fig. 6), an orange-red coloring of the cell interior, due to a metachromatic effect, is revealed. According to the theory of toluidine stains, these orange stained parts mark the cytoplasm, possibly rich in RNA.
Blue stained strands or patches are also visible, especially seen on the image to the right of Fig. 6.
Again according to the theory, these blue regions should mark DNA-rich areas (nuclei) of the cell.
- Obviously, the cell's walls are stained purplish-violet and seem to contain a high content of dye.
This retardation of the dark dye in the cell wall appeared quite disturbing to me, because it obstructed the overall observation of the cell's interior.
Moreover, the tendency to 'over-stain' gave the whole preparation a diffuse, smudged appearance.
So in following staining procedures I refrained from further attempts of toluidine-borax stains.
This should not state that the stain is of no use at all, but certainly more time, a systematic effort and the application of accompanying methods for verification of the molecular foundation for the staining effects should be employed, to give a more differentiated stain.
Possibly, much smaller alga fragments, stained with minute amounts of dye will yield better results.
- Apart from the staining, the image on the left of Fig. 6 highlights another anatomic feature of Cladophora, called pseudo-dichotomous ramification.
Such ramifications occur when branching cells grow out of an intercalary cell.
In opposite to true dichotomy, the filament does not ramify into two new branches, instead each branch is formed by a distinct cell, growing out into a new filament.
Here, at least five branches are depicted.


Fig. 3: Cladophora sp., left: magnification 100x, right: magnification 320x.


Fig. 4: Cladophora sp., left: magnification 100x, right: magnification 400x.


Fig. 5: Cladophora sp., left: magnification 40x, right: magnification 100x, toluidine-borax staining


Fig. 6: Cladophora sp., left: magnification 40x, right: magnification 100x, toluidine-borax staining


Fig. 7: Cladophora sp., left: magnification 40x, right: magnification 100x, picric acid/aniline blue staining
Tue. 01.06.10, Day 5
- staining of Cladophora sp. with chrysoidine and safranine. Afterwards digital imaging of the stained specimen and of the stained objects of the day before (hematoxylin, congo red stainings).

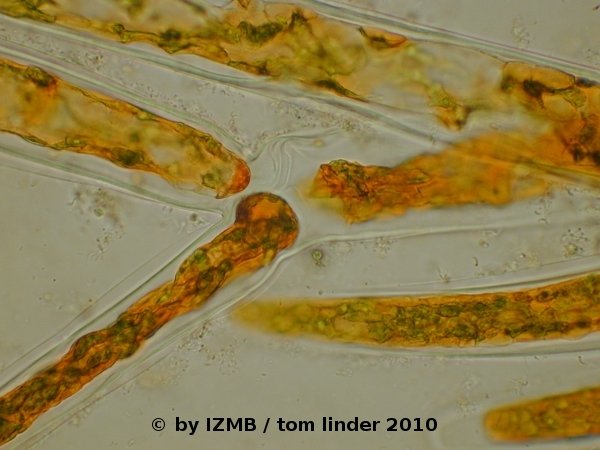
Fig. 08: Cladophora sp., left and right: chrysoidine staining, magnification 400x, left: epiphytic diatoms on the surface of a Cladophora filament
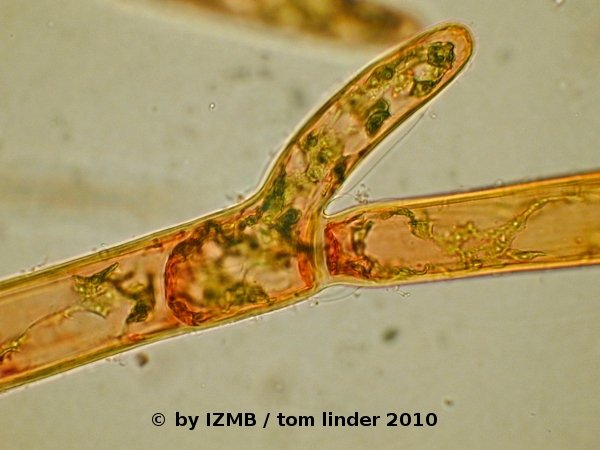
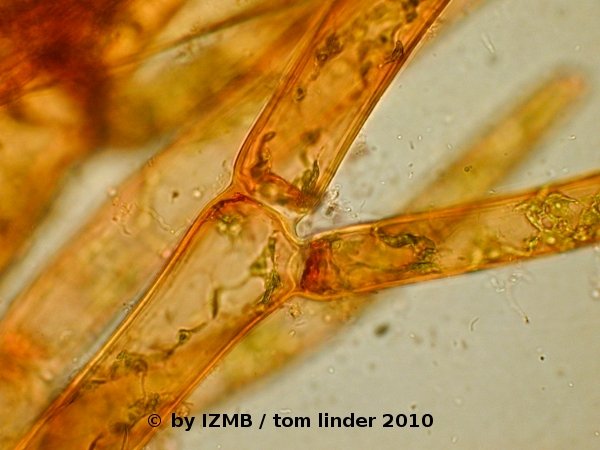
Fig. 09: Cladophora sp., left and right: safranine staining, magnification 400x

Fig. 10: Cladophora sp., safranine staining, magnification 400x, edge of the attaching cell walls visible as a ring in two different planes of focus
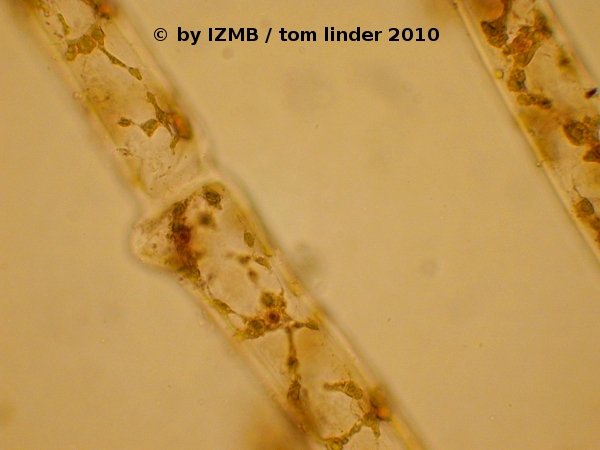
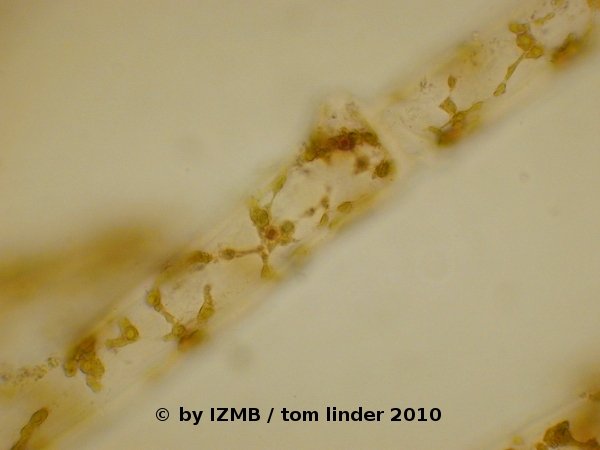
Fig. 11: Cladophora sp., left and right: hematoxylin-alcohol staining (nucleus staining), magnification 400x
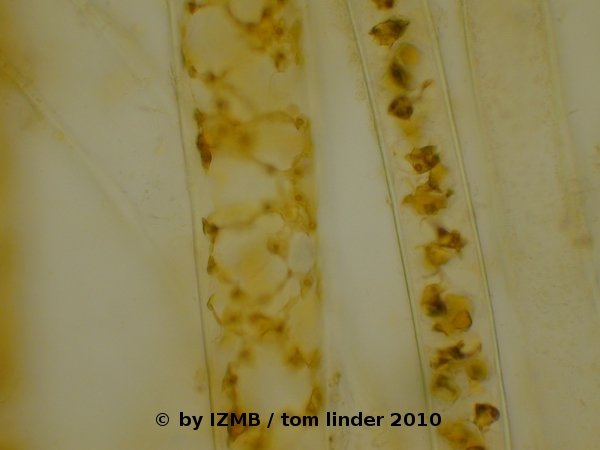

Fig. 12: Cladophora sp., left and right: congo red staining of the cell wall, magnification 400x
- The stainings with hematoxylin/alcohol can be regarded as successful, while the safranine stain showed an accumulation of the dye at the cell's poles
(cell borders of adjacent cells), it should therefore be repeated with a pre-fixation of the cells, as the cells appeared damaged and in particular the cytoplasm
had contracted and appeared deformed. Latter observation was also made on the chrysoidine stained cells. The staining with congo red was unsuccessful,
possibly the dye loses its staining capabilities in salt water.
- staining of Cladophora sp. with chrysoidine and safranine. Afterwards digital imaging of the stained specimen and of the stained objects of the day before (hematoxylin, congo red stainings).


Fig. 08: Cladophora sp., left and right: chrysoidine staining, magnification 400x, left: epiphytic diatoms on the surface of a Cladophora filament


Fig. 09: Cladophora sp., left and right: safranine staining, magnification 400x

Fig. 10: Cladophora sp., safranine staining, magnification 400x, edge of the attaching cell walls visible as a ring in two different planes of focus


Fig. 11: Cladophora sp., left and right: hematoxylin-alcohol staining (nucleus staining), magnification 400x


Fig. 12: Cladophora sp., left and right: congo red staining of the cell wall, magnification 400x
Wed. 02.06.10, Day 6
- Images of another species with a bushy habitus, which could be possibly identified as Enteromorpha clathrata as the habitus and the number of pyrenoids most closely resemble the taxonomic description of this species. Against such finding seems to point its dwarfish growth and the increased density of its ramifications which stand against the description found in Kornmann's field guide. Moreover, the main filaments of the thallus consist of several registers of cells while the branching filaments consists strictly of only two lines, which seems to be characteristic for this species.

Fig. 13: Enteromorpha sp., Habitus

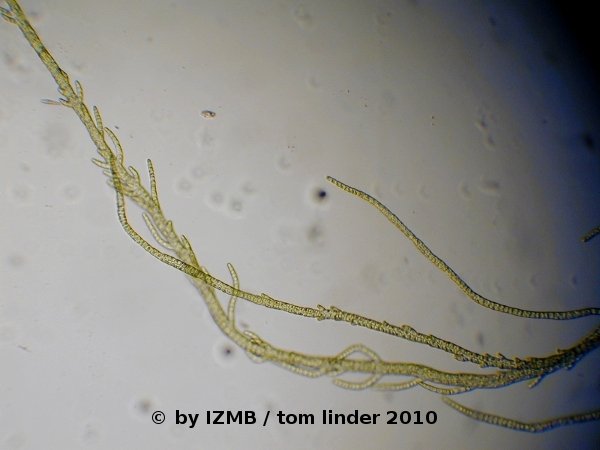
Fig. 14: Enteromorpha sp., magnification 100x, left: main filament with ramifications; right: branching pattern at the tip of a branching filament
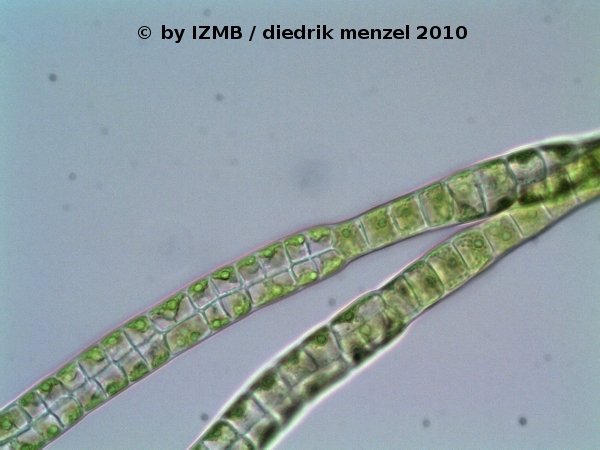
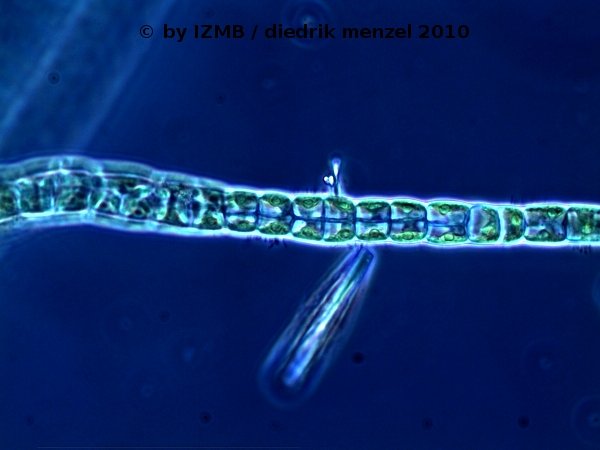
Fig. 15: Enteromorpha sp., magnification 400x, left: branching filament, consisting of two lines of cells, with a twist by 90°;
right: branching filament, consisting of two lines of cells, viewed in phase constrast, which shows the 'napoleon hat'-shaped chloroplasts located at the edge of the cells, Images: Prof. D. Menzel


Fig. 16: Enteromorpha clathrata, magnification 400x
left: multi-registered main filament with 1 or 2 pyrenoids
right: tip of a single-lined filament covered with 'hairy' looking microorganisms (probably bacteria)


Fig. 17: Asparagus sp., wild asparagus

Fig. 18: Orobanche sp., Broomrape


Fig. 19: Sunset, seen from the North of Hiddensee (Dornbusch), viewed through the bushes
- Images of another species with a bushy habitus, which could be possibly identified as Enteromorpha clathrata as the habitus and the number of pyrenoids most closely resemble the taxonomic description of this species. Against such finding seems to point its dwarfish growth and the increased density of its ramifications which stand against the description found in Kornmann's field guide. Moreover, the main filaments of the thallus consist of several registers of cells while the branching filaments consists strictly of only two lines, which seems to be characteristic for this species.

Fig. 13: Enteromorpha sp., Habitus


Fig. 14: Enteromorpha sp., magnification 100x, left: main filament with ramifications; right: branching pattern at the tip of a branching filament


Fig. 15: Enteromorpha sp., magnification 400x, left: branching filament, consisting of two lines of cells, with a twist by 90°;
right: branching filament, consisting of two lines of cells, viewed in phase constrast, which shows the 'napoleon hat'-shaped chloroplasts located at the edge of the cells, Images: Prof. D. Menzel


Fig. 16: Enteromorpha clathrata, magnification 400x
left: multi-registered main filament with 1 or 2 pyrenoids
right: tip of a single-lined filament covered with 'hairy' looking microorganisms (probably bacteria)


Fig. 17: Asparagus sp., wild asparagus

Fig. 18: Orobanche sp., Broomrape


Fig. 19: Sunset, seen from the North of Hiddensee (Dornbusch), viewed through the bushes
Thu. 03.06.10, Day 7
- day off: little trip on foot to the South of the island (ca. 20 km), way down along the coast of the Baltic sea, way back along the 'Bodden'
- taking samples of various Ulvophyceae at the rim of the 'Bodden' south of Neuendorf
- preparation of an intermediate presentation (until 3:30 in the morning !)


Fig. 20: left: green lagae habitat at the shore line of the Baltic sea at the west coast of Hiddensee; right: brown algae habitat at the 'Bodden' shore, east coast of Hiddensee


Fig. 21: left: Enteromorpha habitat in the shallow waters of the Baltic Sea in the South of Hiddensee (Gellen); right: Entermorpha habitat at the shores of the Bodden in the South of Hiddensee (Gellen)


Fig. 22: left: Warning sign in front of the core zone of the National Park 'Vorpommersche Boddenlandschaft' in the South of Hiddensee (Gellen); right: Male cone of a pine, South of Hiddensee (Gellen)


Fig. 23: left: View to the South at the West coast of Hiddensee; right: Pine grove at the West coast of the South of Hiddensee (Gellen), view to the North


Fig. 24: Vegetation in the South of Hiddensee (Gellen), left: grass, probably a Carex species; right: flower of Armeria maritima, sea thrift

Fig. 25: dead male of Vipera berus, the common European adder. These snakes are very common to Hiddensee and there is even a special monitoring project. Anyhow the snakes are toxic and a bite can be dangerous.
- day off: little trip on foot to the South of the island (ca. 20 km), way down along the coast of the Baltic sea, way back along the 'Bodden'
- taking samples of various Ulvophyceae at the rim of the 'Bodden' south of Neuendorf
- preparation of an intermediate presentation (until 3:30 in the morning !)


Fig. 20: left: green lagae habitat at the shore line of the Baltic sea at the west coast of Hiddensee; right: brown algae habitat at the 'Bodden' shore, east coast of Hiddensee


Fig. 21: left: Enteromorpha habitat in the shallow waters of the Baltic Sea in the South of Hiddensee (Gellen); right: Entermorpha habitat at the shores of the Bodden in the South of Hiddensee (Gellen)


Fig. 22: left: Warning sign in front of the core zone of the National Park 'Vorpommersche Boddenlandschaft' in the South of Hiddensee (Gellen); right: Male cone of a pine, South of Hiddensee (Gellen)


Fig. 23: left: View to the South at the West coast of Hiddensee; right: Pine grove at the West coast of the South of Hiddensee (Gellen), view to the North


Fig. 24: Vegetation in the South of Hiddensee (Gellen), left: grass, probably a Carex species; right: flower of Armeria maritima, sea thrift

Fig. 25: dead male of Vipera berus, the common European adder. These snakes are very common to Hiddensee and there is even a special monitoring project. Anyhow the snakes are toxic and a bite can be dangerous.
Fri. 04.06.10, Day 8
- Presentations of the intermediate results
- taking various samples from flora of the Bodden near the port of Kloster, especially collected: Chara, Fucus, Myriophyllum, red algae and samples of the sediment
- imaging of Enteromorpha and staining with KI2 (until 2:00 o'clock in the morning)


Fig. 26: left: Fucus sp. at the shore of the Bodden near the port of Kloster, Hiddensee;
right: Habitus Ulva sp.


Fig. 27: left: Fucus sp. at the shore of the Bodden near the port of Kloster, Hiddensee;
right: Habitat of Chara sp. (Chara youth stage at the shore of the Bodden)


Fig. 28:left: Thallus of Ulva sp. with gas bubbles, magnification 7x;
right: stalk of Cladophora sp., consisting of several filaments twisted around each other
- Presentations of the intermediate results
- taking various samples from flora of the Bodden near the port of Kloster, especially collected: Chara, Fucus, Myriophyllum, red algae and samples of the sediment
- imaging of Enteromorpha and staining with KI2 (until 2:00 o'clock in the morning)


Fig. 26: left: Fucus sp. at the shore of the Bodden near the port of Kloster, Hiddensee;
right: Habitus Ulva sp.


Fig. 27: left: Fucus sp. at the shore of the Bodden near the port of Kloster, Hiddensee;
right: Habitat of Chara sp. (Chara youth stage at the shore of the Bodden)


Fig. 28:left: Thallus of Ulva sp. with gas bubbles, magnification 7x;
right: stalk of Cladophora sp., consisting of several filaments twisted around each other
Sat. 05.06.10, Day 9
- Imaging of Chara, Ulva and Cladophora
- Staining of Cladophora with KI2 and safranine
- The safranine staining (1:100 dilution of stock solution) was performed with a pre-fixation with a solution of ~3,7 % formaldehyde (1:10 dilution of a 37% stock solution), incubated for a few minutes.
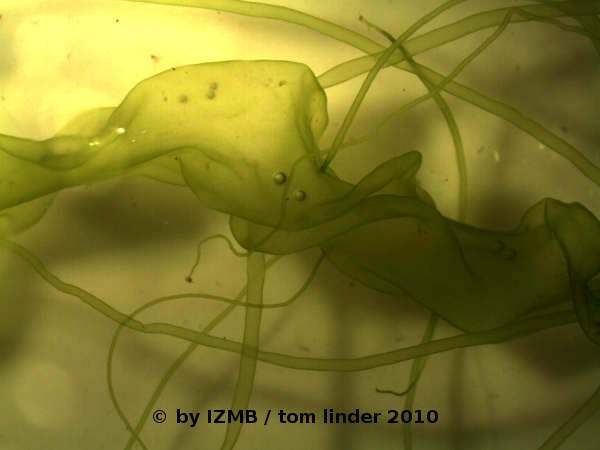
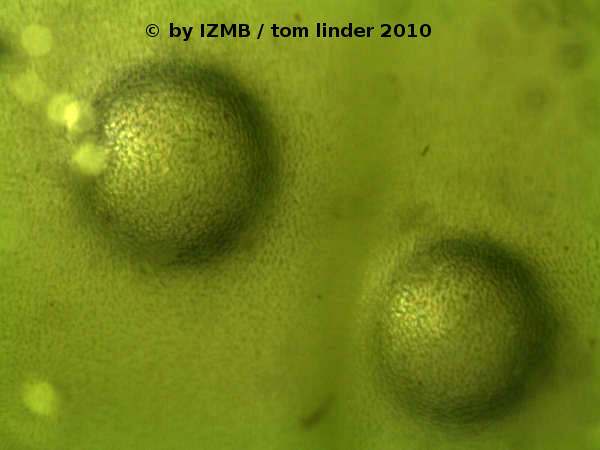
Fig. 29: left: thallus of Enteromorpha intestinalis, magnification 7x; right: Gas inclusions in the thallus of Enteromorpha intestinalis, magnification 90x
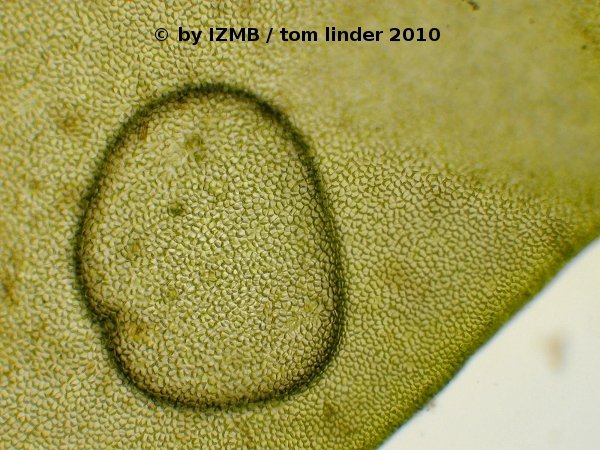

Fig. 30: Gas inclusions in the thallus of Enteromorpha sp., magnification 100x


Fig. 31: Thallus of Enteromorpha sp., magnification 400x


Fig. 32: Lugol staining of the thallus of Enteromorpha sp., the brown-violet coloring may indicate, that amylopectin is the most prominent polysaccharide of the pyrenoid's starch, left: magnification 100x; right: magnification 400x


Fig. 33: Lugol staining of Enteromorpha sp., magnification 400x; left: ripped thallus; right: edge of thallus
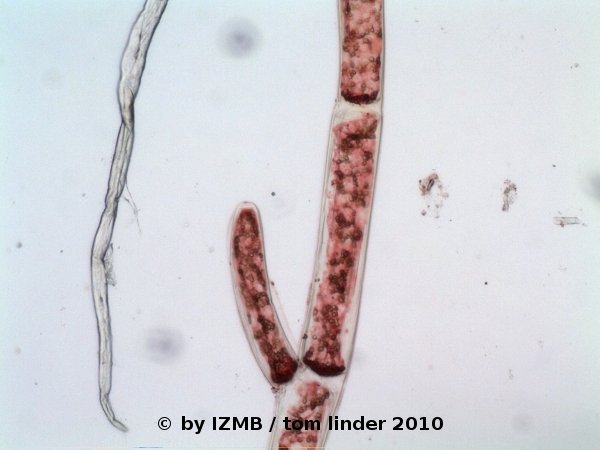
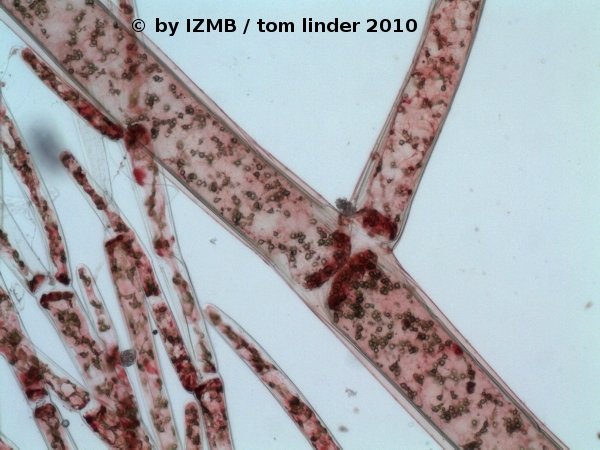
Fig. 34: Safranine staining of Cladophora sp., magnification 100x


Fig. 35: Safranine staining of Cladophora sp., magnification 100x, Nomarsky illumination
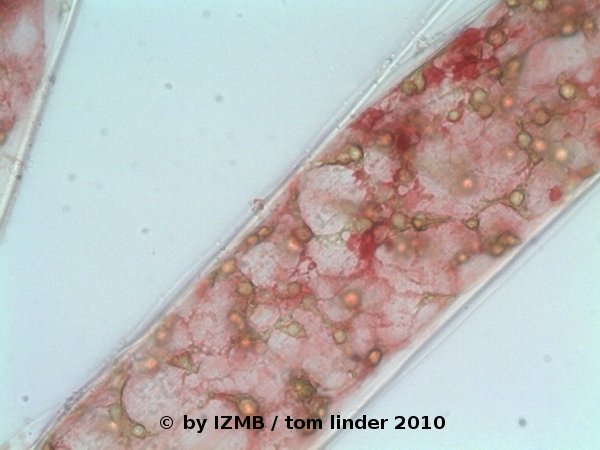
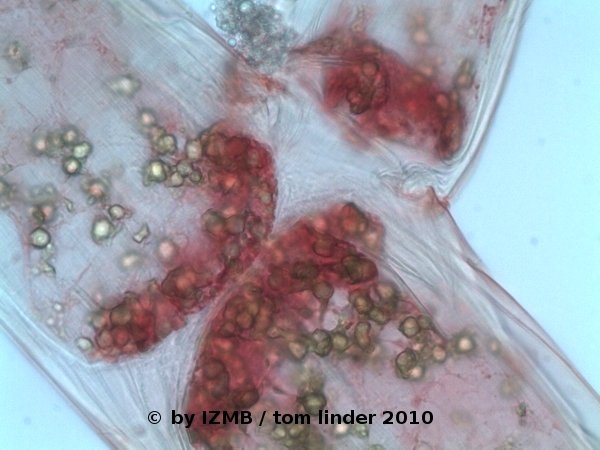
Fig. 36: Safranine staining of Cladophora sp., magnification 400x

Fig. 37: Habitus Chara baltica


Fig. 38: left: Habitus Chara baltica, magnification 7x; right: Rhizoids of Chara baltica, magnification 20x


Fig. 39: left: Detail of a lateral branch ('leaves') of Chara baltica, magnification 90x; right: Rhizoids of Chara baltica, magnification 20x


Fig. 40: left: growing lateral branch of Chara baltica, magnification 40x; right: nodial whorl of Chara baltica, magnification 20x
- Imaging of Chara, Ulva and Cladophora
- Staining of Cladophora with KI2 and safranine
- The safranine staining (1:100 dilution of stock solution) was performed with a pre-fixation with a solution of ~3,7 % formaldehyde (1:10 dilution of a 37% stock solution), incubated for a few minutes.


Fig. 29: left: thallus of Enteromorpha intestinalis, magnification 7x; right: Gas inclusions in the thallus of Enteromorpha intestinalis, magnification 90x


Fig. 30: Gas inclusions in the thallus of Enteromorpha sp., magnification 100x


Fig. 31: Thallus of Enteromorpha sp., magnification 400x


Fig. 32: Lugol staining of the thallus of Enteromorpha sp., the brown-violet coloring may indicate, that amylopectin is the most prominent polysaccharide of the pyrenoid's starch, left: magnification 100x; right: magnification 400x


Fig. 33: Lugol staining of Enteromorpha sp., magnification 400x; left: ripped thallus; right: edge of thallus


Fig. 34: Safranine staining of Cladophora sp., magnification 100x


Fig. 35: Safranine staining of Cladophora sp., magnification 100x, Nomarsky illumination


Fig. 36: Safranine staining of Cladophora sp., magnification 400x

Fig. 37: Habitus Chara baltica


Fig. 38: left: Habitus Chara baltica, magnification 7x; right: Rhizoids of Chara baltica, magnification 20x


Fig. 39: left: Detail of a lateral branch ('leaves') of Chara baltica, magnification 90x; right: Rhizoids of Chara baltica, magnification 20x


Fig. 40: left: growing lateral branch of Chara baltica, magnification 40x; right: nodial whorl of Chara baltica, magnification 20x
Sun. 06.06.10, Day 10
- day off
- little trip to the Altbessin in the North-East of Hiddensee

Fig. 41: Habitus of Enteromorpha sp.


Fig. 42: left: Habitus of Enteromorpha sp.; right: Habitus of Enteromorpha intestinalis


Fig. 43: left: Shores of the Bodden at Altbessin; right: View on the Neubessin

Fig. 44: a caterpillar
- day off
- little trip to the Altbessin in the North-East of Hiddensee

Fig. 41: Habitus of Enteromorpha sp.


Fig. 42: left: Habitus of Enteromorpha sp.; right: Habitus of Enteromorpha intestinalis


Fig. 43: left: Shores of the Bodden at Altbessin; right: View on the Neubessin

Fig. 44: a caterpillar
Mon. 07.06.10, Day 11
- repeated staining of Cladophora with KI2, because the original images were lost
- Imaging of the stained pyrenoids
- Staining of Entermorpha with safranine and a pre-fixation by a ~3,7% formaldehyde solution (1:10 diluted from 37% stock solution), incubated for a few minutes and a fixation with 70% ethanol/15% acetic acid (AAA fixation for Alcohol/Acetic Acid). Moreover a staining with 0,1 % toluidine blue was performed.
- Hematoxylin-alcohol staining of Chara baltica, incubated overnight
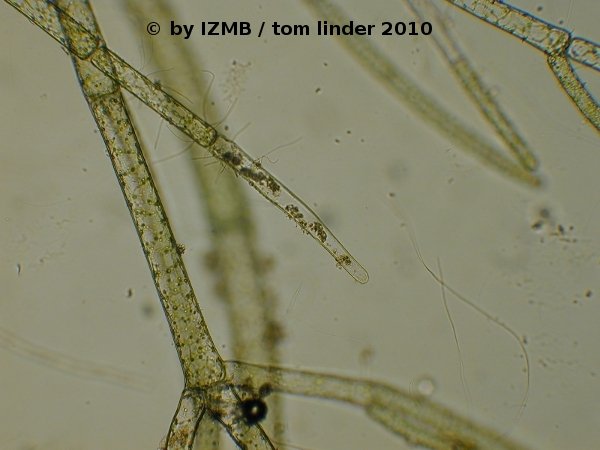

Fig. 45: Epiphytic bacteria on Cladophora sp., left: magnification 400x, right: magnification 100x

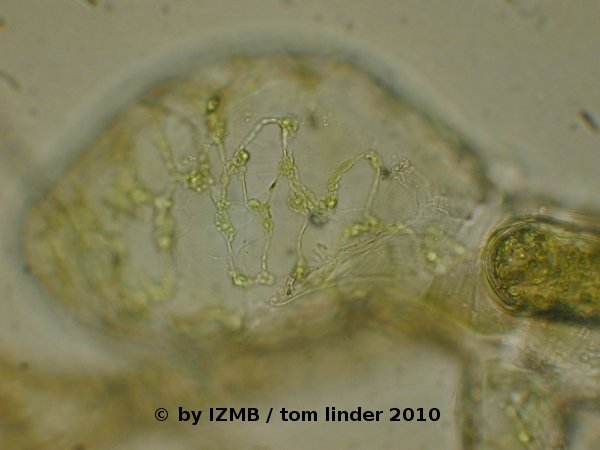
Fig. 46: Thickened basal cell of Cladophora sp., possibly from a wounding, left: magnification 100x, right: magnification 400x
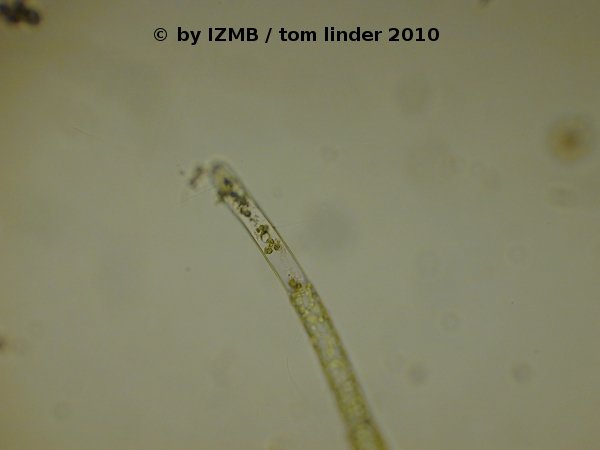
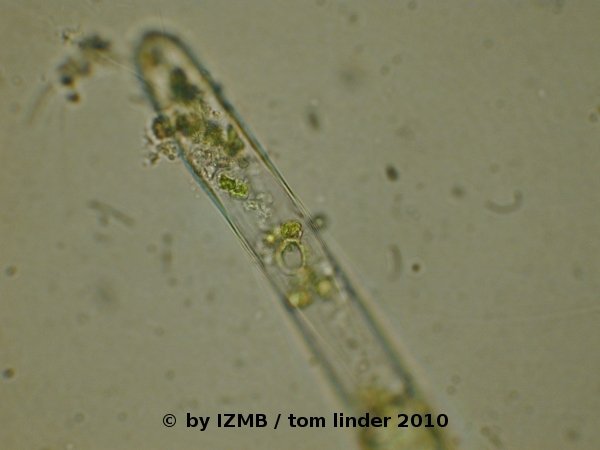
Fig. 47: Circular shaped opening in an emptied sporangium/gametangium of Cladophora sp., left: magnification 100x, right: magnification 400x
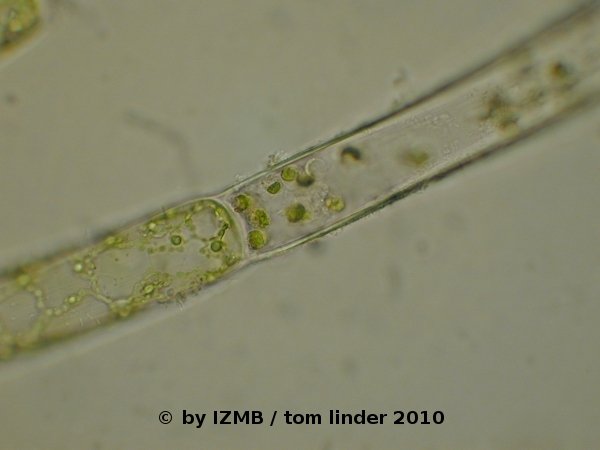

Fig. 48: left: circular opening of an emptied sporangium/gametangium of Cladophora sp., magnification 400x; right: Agglomeration of cells or cytoplasmatic fragments at an emptied cell of Cladophora sp.

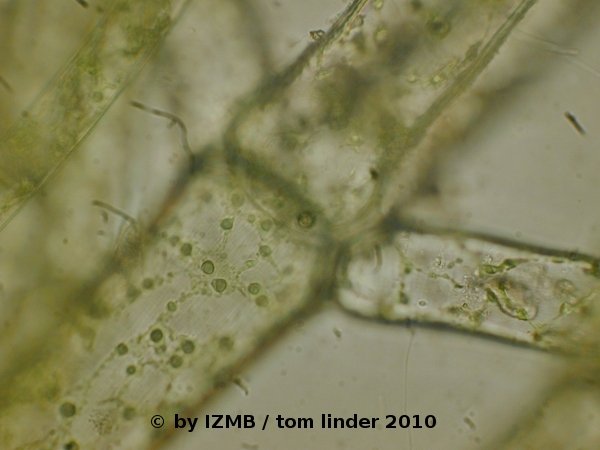
Fig. 49: Lugol staining of Cladophora sp., left: magnification 100x, right: magnification 400x

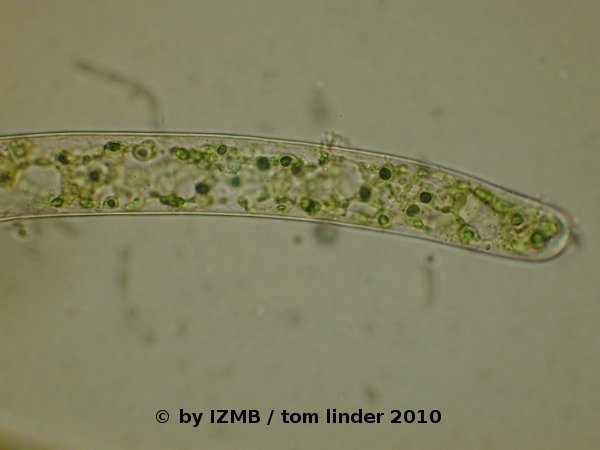
Fig. 50: Lugol staining of Cladophora sp. with stained (starch containing) pyrenoids of the chloroplasts, magnification 400x


Fig. 51: Safranine staining of Enteromorpha sp., fertile thallus, left: magnification 100x, right: magnification 400x
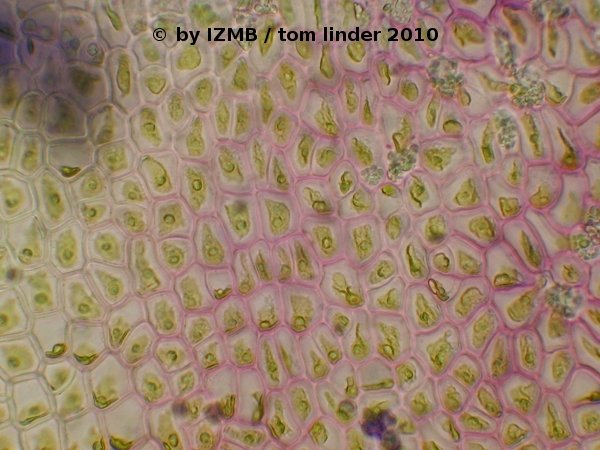
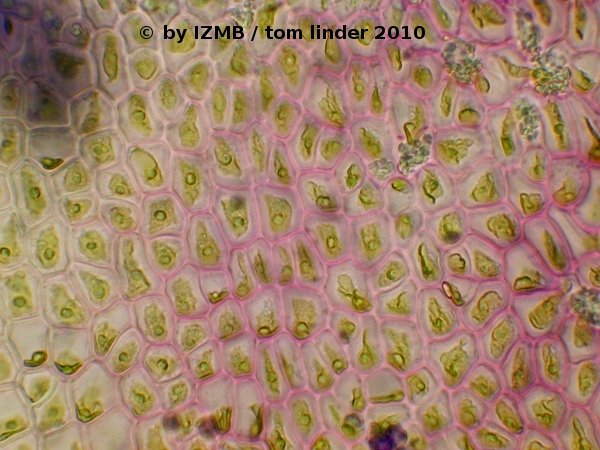
Fig. 52: Toluidine blue staining of Enteromorpha sp., magnification 400x
- repeated staining of Cladophora with KI2, because the original images were lost
- Imaging of the stained pyrenoids
- Staining of Entermorpha with safranine and a pre-fixation by a ~3,7% formaldehyde solution (1:10 diluted from 37% stock solution), incubated for a few minutes and a fixation with 70% ethanol/15% acetic acid (AAA fixation for Alcohol/Acetic Acid). Moreover a staining with 0,1 % toluidine blue was performed.
- Hematoxylin-alcohol staining of Chara baltica, incubated overnight


Fig. 45: Epiphytic bacteria on Cladophora sp., left: magnification 400x, right: magnification 100x


Fig. 46: Thickened basal cell of Cladophora sp., possibly from a wounding, left: magnification 100x, right: magnification 400x


Fig. 47: Circular shaped opening in an emptied sporangium/gametangium of Cladophora sp., left: magnification 100x, right: magnification 400x


Fig. 48: left: circular opening of an emptied sporangium/gametangium of Cladophora sp., magnification 400x; right: Agglomeration of cells or cytoplasmatic fragments at an emptied cell of Cladophora sp.


Fig. 49: Lugol staining of Cladophora sp., left: magnification 100x, right: magnification 400x


Fig. 50: Lugol staining of Cladophora sp. with stained (starch containing) pyrenoids of the chloroplasts, magnification 400x


Fig. 51: Safranine staining of Enteromorpha sp., fertile thallus, left: magnification 100x, right: magnification 400x


Fig. 52: Toluidine blue staining of Enteromorpha sp., magnification 400x
Tue. 08.06.10, Day 12
- Time series of the cytoplasmic streaming in the peripheral, internodal cells of Chara baltica
- Imaging of the hematoxylin-alcohol staining of Chara baltica
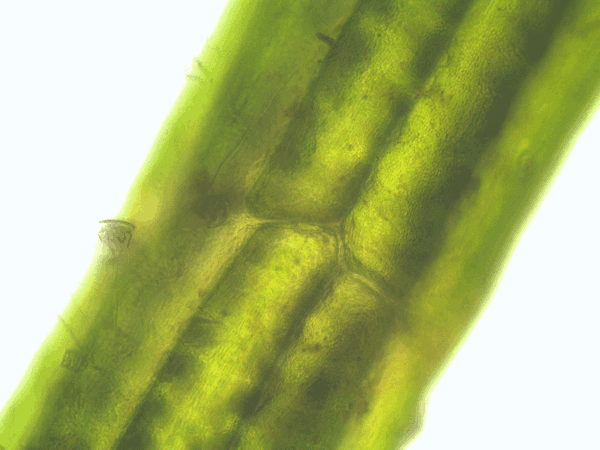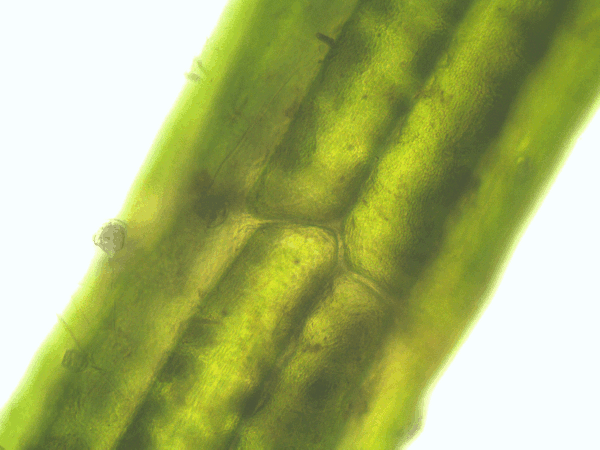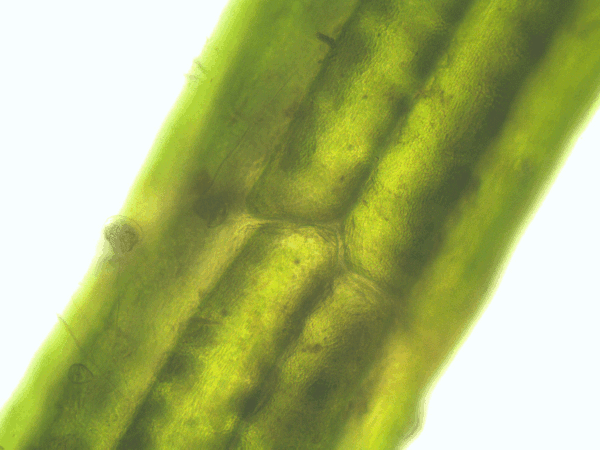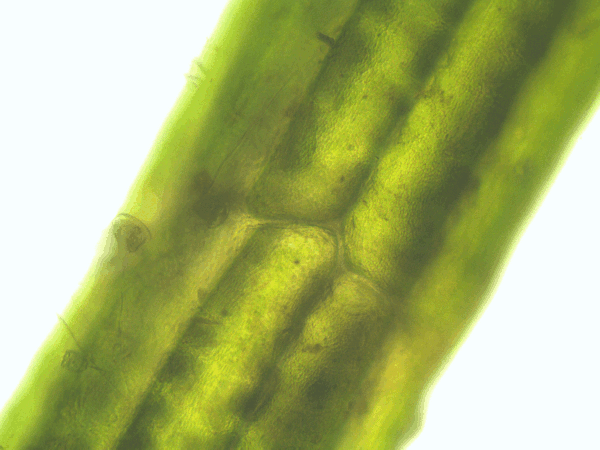
Fig. 53: Animation of the cytoplasmic streaming in the peripheral cells of Chara baltica


Fig. 54: left: Detached lateral branch of Chara baltica, magnification 100x; right: Hematoxylin-Alcohol staining of Chara baltica, showing the stained nuclei

Fig. 55: Montage of the basal region of Enteromorpha sp., magnification 100x, acridine orange staining

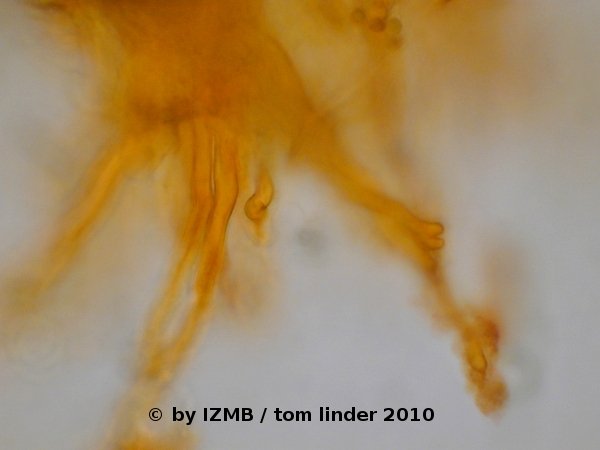
Fig. 56: Acridine orange staining of a rhizoid of Enteromorpha sp., left: magnification 100x, right: magnification 400x
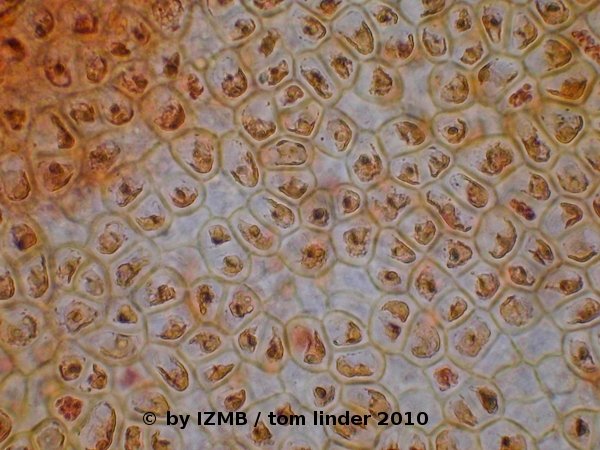
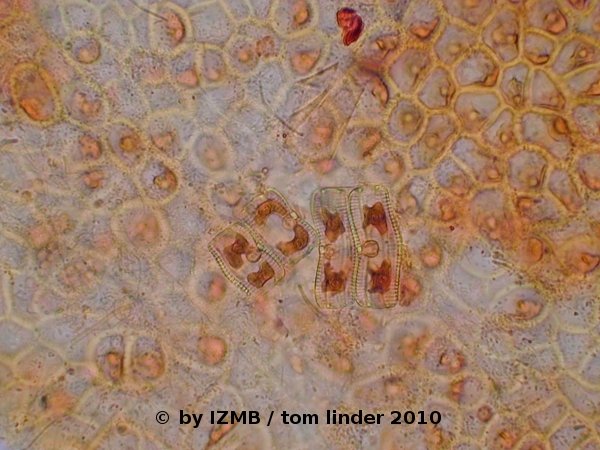
Fig. 57: Safranine staining of Enteromorpha sp., magnification 400x; left: stages of cell division, right: Diatoms
- Time series of the cytoplasmic streaming in the peripheral, internodal cells of Chara baltica
- Imaging of the hematoxylin-alcohol staining of Chara baltica

Fig. 53: Animation of the cytoplasmic streaming in the peripheral cells of Chara baltica


Fig. 54: left: Detached lateral branch of Chara baltica, magnification 100x; right: Hematoxylin-Alcohol staining of Chara baltica, showing the stained nuclei

Fig. 55: Montage of the basal region of Enteromorpha sp., magnification 100x, acridine orange staining


Fig. 56: Acridine orange staining of a rhizoid of Enteromorpha sp., left: magnification 100x, right: magnification 400x


Fig. 57: Safranine staining of Enteromorpha sp., magnification 400x; left: stages of cell division, right: Diatoms
Wed. 09.06.10, Day 13
- Excursion to the heath south of Vitte with Mrs. Dr. Blindow
- Imaging of the hematoxylin-alcohol staining of Chara baltica and Enteromorpha sp.
- Imaging of Cladophora sp.


Fig. 58: Typical species of the bush vegetation in the protected heath area south of Vitte, Hiddensee; left: unripe berries of the black crowberry, Empetrum nigrum, right: Twigs of the Creeping Willow, Salix repens


Fig. 59: Typical species of the shrub vegetation in the protected heath area south of Vitte, Hiddensee; left: Common heather or ling, Calluna vulgaris, which lends its name together with the other species of heath, Erica, to the landscape of the heathland, right: Flowers of an uprising cross-leaved heath, Erica tetralix, within an ensemble of Common hair moss (Great Goldilocks), Polytrichum commune


Fig. 60: left and right: Round-leaved sundew, Drosera rotundifolia in one of the water filled depressions in the heathland south of Vitte, Hiddensee

Fig. 61: one of the numerous of rattle plants, possibly Rhinanthus serotinus, on a meadow adjacent to a protected area, south of Vitte, Hiddensee
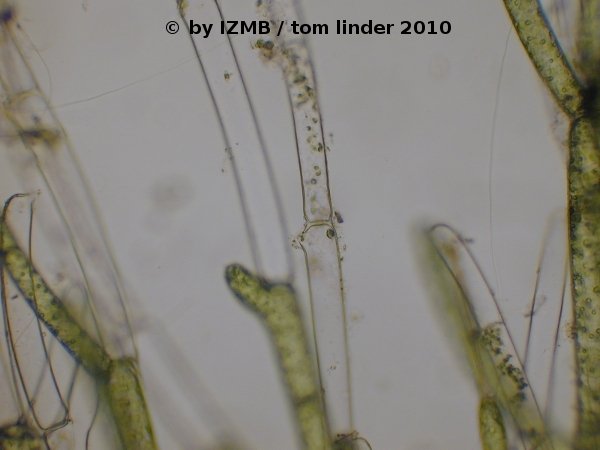

Fig. 62: Opening at the reproductive cells of Cladophora sp., left: magnification 100x, right: magnification 400x


Fig. 63: Main filament of Cladophora sp., left and right: magnification 100x
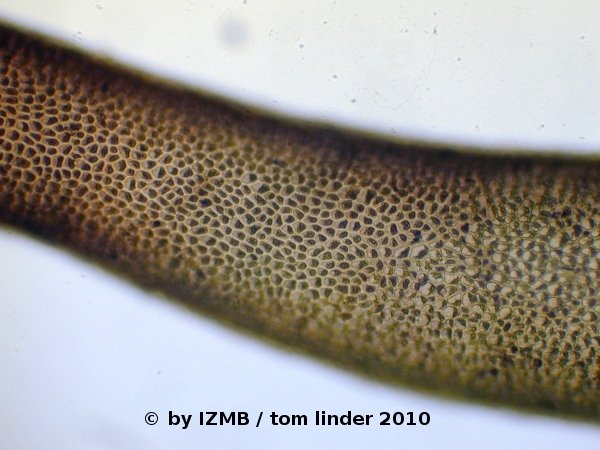

Fig. 64: Hematoxylin staining of Enteromorpha sp., left: magnification 100x, right: magnification 400x
- Excursion to the heath south of Vitte with Mrs. Dr. Blindow
- Imaging of the hematoxylin-alcohol staining of Chara baltica and Enteromorpha sp.
- Imaging of Cladophora sp.


Fig. 58: Typical species of the bush vegetation in the protected heath area south of Vitte, Hiddensee; left: unripe berries of the black crowberry, Empetrum nigrum, right: Twigs of the Creeping Willow, Salix repens


Fig. 59: Typical species of the shrub vegetation in the protected heath area south of Vitte, Hiddensee; left: Common heather or ling, Calluna vulgaris, which lends its name together with the other species of heath, Erica, to the landscape of the heathland, right: Flowers of an uprising cross-leaved heath, Erica tetralix, within an ensemble of Common hair moss (Great Goldilocks), Polytrichum commune


Fig. 60: left and right: Round-leaved sundew, Drosera rotundifolia in one of the water filled depressions in the heathland south of Vitte, Hiddensee

Fig. 61: one of the numerous of rattle plants, possibly Rhinanthus serotinus, on a meadow adjacent to a protected area, south of Vitte, Hiddensee


Fig. 62: Opening at the reproductive cells of Cladophora sp., left: magnification 100x, right: magnification 400x


Fig. 63: Main filament of Cladophora sp., left and right: magnification 100x


Fig. 64: Hematoxylin staining of Enteromorpha sp., left: magnification 100x, right: magnification 400x